Products
Products
Nephron Pharmaceuticals Corporation is a leading manufacturer of inhalation products, sterile pre-filled medications, & more.
Affordable, High-Quality Healthcare Solutions
Affordable, high-quality healthcare solutions
We produce affordable, high-quality inhalation solutions and suspension products that treat respiratory conditions, and we are the only manufacturer of Racemic Epinephrine, 2.25%, 0.5mL.
We value our customers and have longstanding relationships with major drug wholesalers that distribute our products to hospitals, retail pharmacies, mail order pharmacies, home care companies, and long-term care facilities.
Respiratory Therapy
Nephron Pharmaceuticals Corporation is an industry leading manufacturer of sterile, preservative-free respiratory inhalation medications. Our customers include hospitals, retail pharmacies, home care companies, long term care facilities, and mail order pharmacies. All of our medications are available individually wrapped and barcoded for ease of use during bedside scanning. Nephron products are proudly made in the USA!
Respiratory Therapy
Inhalation Solutions
Inhalation Suspensions
503B Outsourcing
The Nephron 503B Outsourcing Facility is equipped to manufacture products that are in short supply under Current Good Manufacturing Practice (cGMP) conditions. In an effort to alleviate the ongoing drug shortages in the US drug supply chain, Nephron has responded with 24-7 production of shortage products packaged in prefilled syringes and sterile small-volume parenteral solutions (SVPs). "We will do everything in our power to prevent the delay of necessary patient treatments, and continue to develop shortage list products, with a focus on quality and sterile manufacturing," said Lou Kennedy, CEO.
503B Outsourcing
Pre-Filled Syringes
IV Bags
Plastic Luer-Lock Vials
Register for the Nephron 503B Online Ordering Portal
Register today to order Nephron 503B Outsourcing products. Registration will provide your facility access to the Nephron 503B ordering portal, for easy online ordering. The process is quick and simple!
Ready to start your account? Please contact us at NOFaccounts@nephronpharm.com
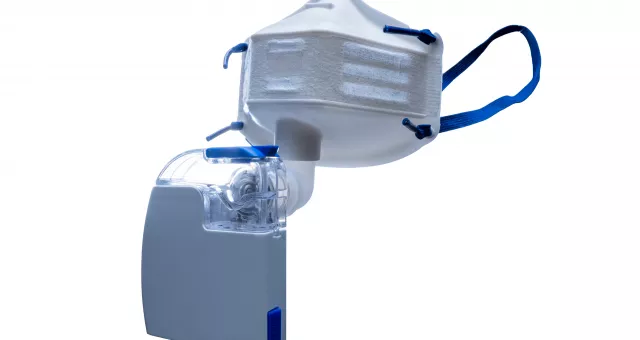
Introducing the New X-HALE Mask
Want to reduce caregivers' risk of exposure while treating patients? Then the new X-HALE filtered aerosol mask is for you. It works with both the Pocket Neb™ (a lightweight, rechargeable vibrating mesh nebulizer for aerosol drug delivery) and standard nebulizer kits to filter out patients' exhaled bioaerosols.
Learn More
'Works with KitCheck' Product Available
Nephron offers RFID-enabled products which are out-of-the-box, ready for use, with KitCheck, Bluesight, Inc.’s, Intelligence Solution Product. This RFID technology provides visibility into the unit dose of the medication lifecycle, decreases stockouts, increases staff efficiency, and improves patient safety.
The “Works with KitCheck” certification is designed for your pharmacy and hospital to easily recognize product is compatible with KitCheck.
Sterile Water, Sterile Products!
As a leader in sterile pharmaceutical manufacturing, our cGMP compliant facilities boast a high purity water system that is reliable and capable of consistently providing Water for Injection (WFI) to meet the established standards of purity. The components of our high purity water system have been designed with the quality of the water supplied to the plant in mind. Once city water enters our facilities, it flows into the pre-treatment system. This involves sand filtration, carbon beds, reverse osmosis, UV treatments and deionization.
After water completes the pre-treatment process, it goes through two Multiple Effect Water Stills which use a staged evaporation and condensation process to produce Water for Injection. Once produced, this Water for Injection enters our storage and distribution system. The storage/distribution system is constructed completely of 316L stainless steel and includes two 6,000 gallon tanks, distribution pumps, and piping.
Water for Injection is stored in a continuously circulating system and is maintained at no less than 75 °C and is thus self-sanitizing. Our system is designed to eliminate “dead legs,” thereby avoiding the growth of bacteria. Samples are taken daily at multiple points throughout our system to insure the quality of the Water for Injection. These samples are collected by Environmental Monitoring Technicians and are tested by both our Microbiology and Chemistry laboratories.